When a product leaves a manufacturing facility, it shouldn’t carry invisible threats. But in food plants, pharmaceutical labs, and cosmetic production lines, contamination isn’t always visible. A single bacterium on a conveyor belt, a mold spore in the air, or metal particles from worn equipment can ruin batches, trigger recalls, or worse - make people sick. That’s why environmental monitoring isn’t optional. It’s the frontline defense against contamination before it ever touches your product.
What Environmental Monitoring Actually Does
Environmental monitoring (EM) is the routine testing of surfaces, air, water, and equipment in a facility to detect contaminants before they compromise products. It’s not about checking the final product - it’s about checking the environment that makes the product. The goal? Catch problems early, stop them from spreading, and prove your controls are working.
The U.S. FDA and the European Medicines Agency don’t treat this as a suggestion. They require it. In pharmaceuticals, EU GMP Annex 1 (updated in 2023) mandates continuous air monitoring in cleanrooms. In food production, USDA’s Listeria Rule (9 CFR part 430) forces RTE (ready-to-eat) facilities to test for Listeria monocytogenes weekly on food contact surfaces. These aren’t paperwork exercises. They’re legal obligations backed by real consequences: shutdowns, fines, and lost trust.
According to CDC data, 87% of foodborne illness outbreaks tied to environmental sources could have been prevented with proper monitoring. That’s not just a statistic - it’s a warning. Every recall costs money. Every illness costs lives. Environmental monitoring is the cheapest insurance you can buy.
The Zone System: Risk-Based Sampling
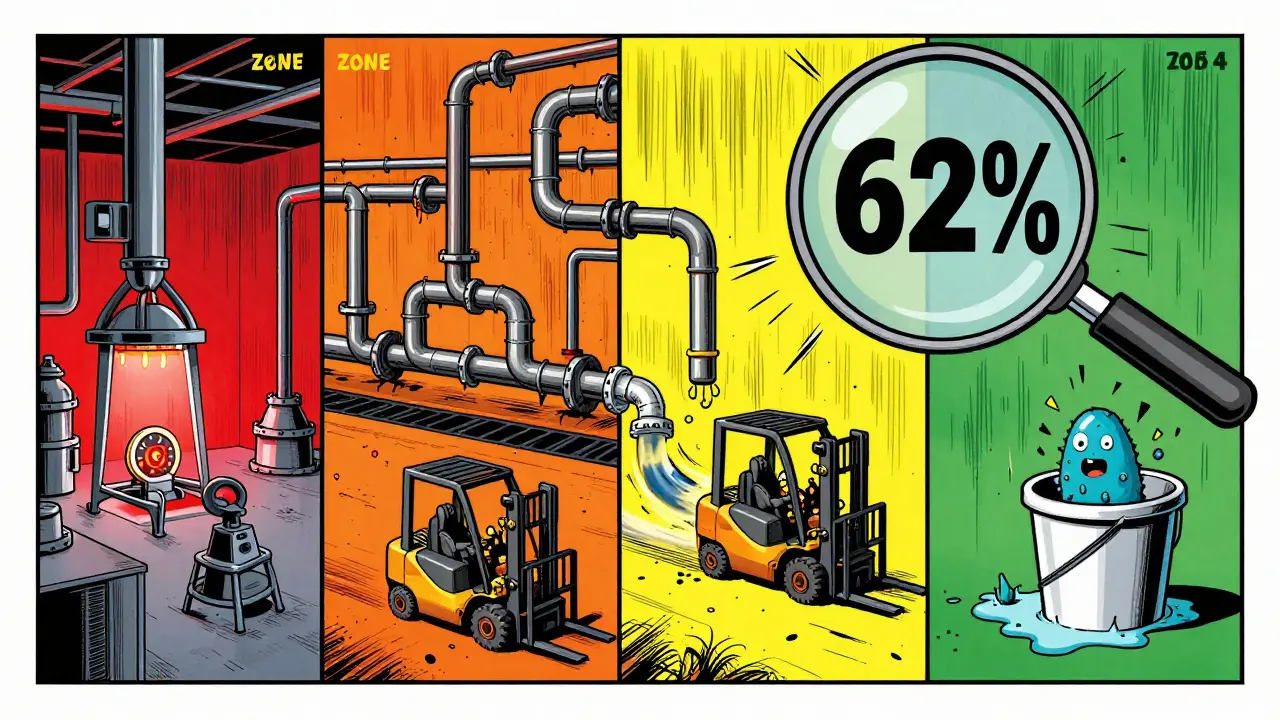
Not all surfaces are created equal. That’s why every serious facility uses the zone classification system. It divides your facility into four risk levels, so you focus your testing where it matters most.
- Zone 1: Direct food or product contact surfaces - slicers, mixers, filling nozzles, packaging rollers. These are high-risk. You test these daily or every shift.
- Zone 2: Non-product contact surfaces near Zone 1 - equipment housings, refrigeration units, nearby walls. These can splash or shed particles. Test weekly.
- Zone 3: Remote but still inside the production area - forklifts, carts, overhead pipes, drains. Surprising fact: a 2010-2013 PPD Laboratories study found that 62% of all contamination events came from Zone 3 and 4 surfaces, not Zone 1. Floors and drains are silent killers.
- Zone 4: Outside the production area - offices, break rooms, storage halls. Test monthly or quarterly, but don’t ignore them. A dirty mop bucket in Zone 4 can still track contaminants into Zone 3.
Here’s the catch: different facilities classify zones differently. One plant might treat an overhead pipe as Zone 1 because it drips condensation onto packaging. Another might call it Zone 3. That’s why risk assessments must be facility-specific. There’s no one-size-fits-all map.
How Testing Works: Tools and Methods
Testing isn’t just swabbing surfaces and sending them off. Each contaminant needs its own method.
- Microbial testing: Swabs or contact plates collect bacteria, yeast, or mold. Results take 24-72 hours. The FDA targets Salmonella and Listeria in food plants. Pharma labs watch for Pseudomonas and Staphylococcus.
- Air sampling: Liquid impingers or solid impactors pull air through a device to capture airborne particles. Results are measured in CFU/m³ (colony-forming units per cubic meter). Continuous monitors in cleanrooms track particles in real time.
- ATP testing: This isn’t microbiological - it’s a quick check for organic residue. ATP swabs glow when they detect biological material. Results in seconds. Facilities using ATP report 32% faster production turnarounds because they don’t wait for lab results to restart lines.
- Water testing: Pharma uses TOC (total organic carbon) and conductivity tests to meet USP <645> standards. Food plants check municipal water against EPA rules.
- Chemical and metal testing: ICP (inductively coupled plasma) detects trace metals. HPLC or GC identifies chemical residues from cleaners or lubricants.
One mistake most facilities make? Mixing up their testing methods. ATP, microbiological, and allergen tests often run in separate systems with no shared data. That’s like having three different dashboards for your car’s engine, fuel, and brakes - you’ll miss the big picture.
Who’s Doing It Right - And Who’s Falling Behind
Pharmaceutical companies lead the pack. 98% have formal environmental monitoring programs. Why? Because a single contaminated vial can trigger a global recall. They monitor temperature, humidity, and air pressure 24/7. Their cleanrooms are ISO Class 5 (EU Grade B) - meaning fewer than 3,520 particles per cubic meter of air.
Food facilities lag. Only 76% have full programs. Many small plants still rely on visual inspections. That’s dangerous. The USDA’s 2023 EMP guide says Listeria can survive for months in drains and cracks. One slip-up - like not cleaning a conveyor joint - can lead to an outbreak.
Here’s a real example: a mid-sized meat processor in Wisconsin was failing Listeria tests in Zone 2. They assumed it was the slicer. Turns out, it was the condensation dripping from an uncleaned overhead pipe (Zone 3). Once they cleaned that pipe and started weekly swabs, contamination dropped to zero.
Even more telling: a PPD study comparing U.S. and Irish facilities found contamination rates were consistently below 0.01% - not because one country was better, but because both followed the same zone-based protocols. Standardization works.
Common Mistakes That Cost Millions
It’s not lack of tools that fails facilities - it’s lack of discipline.
- Inconsistent sampling technique: 68% of facilities admit their staff swab differently each time. Some press hard. Others barely touch. The FDA says sterile swabs must be used correctly - otherwise, you’re not testing the environment. You’re testing your technique.
- Bad zone classification: 42% of facilities have no clear, written zone map. Employees guess. Auditors fail them.
- Ignoring Zone 3 and 4: Floors, drains, and carts get skipped because they’re “not product contact.” But they’re the source of 62% of alerts. That’s not negligence - it’s ignorance.
- No training: The FDA recommends 40 hours of hands-on training before anyone touches a swab. Many facilities give a 10-minute demo. That’s a liability waiting to happen.
- Ignoring data integration: If your ATP results don’t talk to your microbial reports, you’re flying blind. AI-powered analytics are now combining all data streams to predict contamination risks before they happen.

Costs, Compliance, and ROI
Yes, environmental monitoring costs money. Medium-sized food plants spend $15,000-$25,000 a year on supplies and lab fees. They need 2-3 full-time staff. But compare that to the cost of a recall: the USDA says foodborne illness outbreaks cost the U.S. $77.7 billion annually. A single recall can cost $10 million - and destroy a brand.
ROI is clear: facilities using ATP testing cut downtime by 32%. Those with integrated data systems reduce false positives by 27%. And with new FDA guidelines pushing for real-time monitoring and AI-driven trend analysis, the cost of NOT upgrading is rising fast.
The global EM market is projected to hit $12.5 billion by 2027. Growth is driven by stricter regulations - not just in pharma, but in food, cosmetics, and even medical devices. If you’re not investing in EM, you’re betting your business on luck.
What’s Next: The Future of Contamination Control
Environmental monitoring is evolving. The FDA’s 2023 draft guidance encourages next-generation sequencing (NGS) to identify microbes in under 24 hours instead of 72. AI is already being used to predict contamination spikes based on weather, foot traffic, or maintenance schedules.
Antimicrobial resistance is a growing concern. The CDC found 19% of Listeria strains from food environments now resist multiple antibiotics. That means even if you kill most bacteria, the survivors could be dangerous.
By 2027, 38% of monitoring systems will be AI-integrated. That doesn’t mean robots will replace people. It means people will spend less time chasing false alarms and more time fixing real problems.
The bottom line? Environmental monitoring isn’t about compliance. It’s about control. It’s about knowing your facility is clean - not hoping it is.
What is the main purpose of environmental monitoring in manufacturing?
The main purpose is to detect and control contamination - like bacteria, mold, particles, or chemicals - before it reaches the product. It’s a proactive system that prevents recalls, protects consumer health, and proves your facility meets regulatory standards. It’s not about testing the final product; it’s about ensuring the environment where it’s made is safe.
How often should environmental samples be taken?
Frequency depends on the zone and industry. Zone 1 (direct product contact) is tested daily to weekly. Zone 2 (near contact) is tested weekly to monthly. Zone 3 and 4 (remote areas) are tested monthly to quarterly. FDA requires weekly Listeria testing in RTE food facilities. Pharma cleanrooms require continuous air monitoring. Risk-based schedules are key - more frequent where contamination risk is highest.
What’s the difference between ATP testing and microbial testing?
ATP testing detects organic residue - anything biological - using a light reaction. It gives results in seconds and tells you if a surface is clean, but not what’s on it. Microbial testing identifies specific bacteria, mold, or yeast. It takes 24-72 hours but tells you exactly what organism is present. ATP is for quick sanitation checks. Microbial testing is for compliance and root cause analysis.
Why are Zone 3 and 4 surfaces so important?
Even though they’re not direct contact surfaces, Zone 3 and 4 areas - like floors, drains, and carts - are the source of 62% of contamination events, according to PPD Laboratories. Contaminants spread from these areas through air, water, or people’s shoes. Ignoring them is like locking your front door but leaving your back window open.
What industries are most affected by environmental monitoring regulations?
Pharmaceuticals are the most strictly regulated, with EU GMP Annex 1 and FDA requirements for cleanrooms. Food processing, especially ready-to-eat products, is next, under USDA’s Listeria Rule. Cosmetics and medical device manufacturers also face strict standards. All are required to have documented, risk-based environmental monitoring programs to remain compliant.
Can small manufacturing facilities afford environmental monitoring?
Yes, but they often struggle. Only 48% of small facilities (under 50 employees) have fully compliant programs, per USDA data. They can start small: focus on Zone 1 and 2, use ATP swabs for quick checks, partner with a local lab for microbial testing, and train staff properly. The cost of a single recall far exceeds the cost of a basic program. Prioritize risk, not budget.


Amy Lesleighter (Wales) 25.12.2025
i just wish more small shops knew this stuff. zone 3 is where the real trouble hides. my cousin runs a bakery and they ignored the drain for years. one day bam - listeria. all because someone thought "it's not touching the bread". dumb.
Becky Baker 25.12.2025
this is why america needs to stop outsourcing everything. if we kept manufacturing here we'd actually care about clean rooms. europe's got their heads on straight. we're still letting mom and pop shops wing it with paper towels and hope.
Sophia Daniels 25.12.2025
oh sweet jesus. 62% of contamination comes from FLOOR DRAINS AND FORKLIFTS?? i mean... wow. we're spending millions on fancy sensors while someone's muddy boots track mold from the parking lot into zone 3. it's like having a vault with a broken lock and then yelling at the guy who forgot to close the window. the real villain isn't bacteria - it's laziness dressed up as "we're too busy".
Peter sullen 25.12.2025
It is imperative to note, however, that the implementation of a robust, risk-based, zone-classified environmental monitoring protocol-aligned with ISO 14644-1 and FDA 21 CFR Part 11-constitutes not merely a regulatory compliance imperative, but a foundational pillar of operational integrity. Without real-time, data-integrated analytics, one cannot achieve true process control.
Steven Destiny 25.12.2025
if you're not using ATP swabs daily, you're gambling with people's lives. period. stop pretending "visual checks" are enough. that's 1990s thinking. get with the program or shut down.
Fabio Raphael 25.12.2025
i really appreciate how this breaks down zones. i work in a small cosmetic lab and we never thought about drains being a problem. now i'm going to ask my boss to map out our zones properly. maybe we can avoid a recall someday.
Erwin Asilom 25.12.2025
The data supporting zone-based sampling is unequivocal. Consistent methodology, documented protocols, and staff training are non-negotiable. Failure to standardize swab pressure, sampling frequency, or data logging introduces unacceptable variability into the monitoring process.
Sumler Luu 25.12.2025
I'm curious how this applies to home-based food producers. My aunt makes jam in her kitchen. She wipes counters with vinegar. Is that enough? I don't want to sound judgmental, but I worry.
sakshi nagpal 25.12.2025
In India, we have many small pharma units that struggle with this. But I've seen some amazing grassroots efforts-local labs teaching farmers to test water for pesticides using simple kits. Maybe the answer isn't just big tech, but community knowledge.
Sandeep Jain 25.12.2025
zone 3 is real. i work in a plant and no one cares about the floor till someone slips and breaks their ankle. then everyone's like oh wow we should clean that. but the mold? still there. dumb.
roger dalomba 25.12.2025
So we pay $25k a year to test for bacteria... while CEOs fly private to Bermuda. What a world.